The biology of information processing: links from single cells to complex circuits
One of the major challenges in the contemporary neurosciences is the transfer of the increasingly detailed knowledge of cellular processes onto more complex functional, and systemic levels. The auditory system provides an ideal example of such a transition, because it is characterized by a rather unique structure-function relationship of neural arrangements as well as the clear segregation into different anatomical and functional, parallel pathways. In general, acoustic cues carry a multitude of information in the temporal domain and, therefore, the rules and mechanisms underlying the processing of such temporal information can be particularly well studied in the auditory brainstem.
All figures show the neuronal circuit responsible for the detection of interaural time differences (ITD).
Calix of Held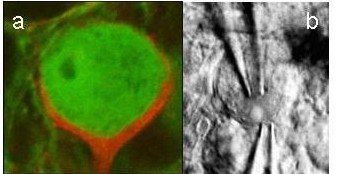 : a) Presynaptic terminal (red) and postsynaptic cell in the MNTB, which provides inhibitory projections to the ITD detector neurons. b) Electrodes during a simultaneous patch-clamp recording from the presynaptic terminal and MNTB soma (both figures provided by Felix Felmy).
: a) Presynaptic terminal (red) and postsynaptic cell in the MNTB, which provides inhibitory projections to the ITD detector neurons. b) Electrodes during a simultaneous patch-clamp recording from the presynaptic terminal and MNTB soma (both figures provided by Felix Felmy).
One structure in the mammalian auditory brainstem most obviously adapted for very precise temporal processing is the pathway that includes the medial nucleus of the trapezoid body (MNTB, Fig. c). MNTB neurons receive their excitatory input via the calyx of Held (Fig. a), one of the largest, without doubt the most compact, probably the fastest, and definitely the temporally most secure chemical synapse known in the vertebrate brain. The anatomy of this synapse allows the combination of simultaneous pre- and post synaptic patch-clamp recordings (Fig. b) with newest imaging methods.
Neuronal Circuits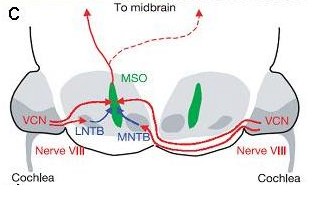 in the auditory brainstem: c) The neuronal circuit comparing the time of arrival (interaural time difference, ITD) in the mammalian auditory brainstem, with one excitatory (red) and one inhibitory (blue) input from each side terminating in the medial superior olive (MSO).
in the auditory brainstem: c) The neuronal circuit comparing the time of arrival (interaural time difference, ITD) in the mammalian auditory brainstem, with one excitatory (red) and one inhibitory (blue) input from each side terminating in the medial superior olive (MSO).
This possibility provided insights into the basic mechanism of synaptic transmission in hitherto unknown detail, in particular, into the regulatory role of calcium in synaptic vesicle release and short-term plasticity. Now knowledge from this specialization can be transferred to the in vivo function of the neural circuit in which it is embedded.
Interestingly, the calyx of Held drives inhibitory neurons and connects the bushy cell fibers from the cochlear nucleus, faithfully conveying information about the precise temporal occurrence and the temporal structure of a sound to the principal cells of the MNTB. The MNTB in turn inhibits a number of auditory centers involved in processing temporal information.
However until recently the importance of this precise timing and, hence, why the MNTB is one of the most prominent structures in our brain, was entirely unclear. The MNTB output has become a model system that has changed our view of how specific and precise inhibitory connections can operate and play a pivotal role in precise temporal processing, even in the sub-millisecond time range. Such a role was not expected, since inhibition has traditionally been thought to be comparably slow and sluggish, and not suited to act on a millisecond-by-millisecond basis.
d) Spike rate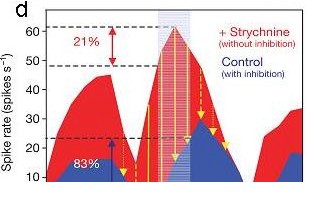 of an MSO cell in response to different ITDs with intact inhibitory inputs (blue) and during pharmacological blockade of inhibition with strychnine (red). Note that inhibitory inputs adjust the steepest slope (best coding) of the ITD function close to 0 ITD. Shaded area indicates the physiologically relevant range of ITDs for the experimental animal used, a Mongolian gerbil (Brand et al., Nature, 2002).
of an MSO cell in response to different ITDs with intact inhibitory inputs (blue) and during pharmacological blockade of inhibition with strychnine (red). Note that inhibitory inputs adjust the steepest slope (best coding) of the ITD function close to 0 ITD. Shaded area indicates the physiologically relevant range of ITDs for the experimental animal used, a Mongolian gerbil (Brand et al., Nature, 2002).





