Phagocytes in the brain: Good or bad?
Neurodegenerative diseases
31.05.2017
The role of microglial cells in neurodegenerative disease is not fully understood. But new results from researchers in Munich and Basel suggest that stimulation of this arm of the immune system might well delay the onset of such disorders.
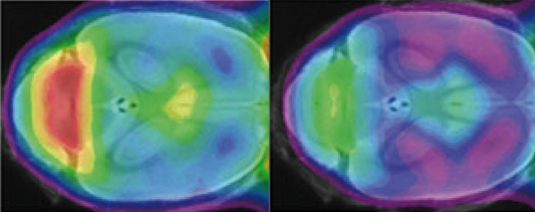
A minimal change in the TREM2 gene results in a marked reduction in the phagocytic activity of microglial cells in the brain of mutant mice (green, on the right) relative to the control (yellow and red, on the left). Source: Haass Lab.
(source: LMU press releases)
The precise impact of the microglia in neurodegenerative diseases such as Alzheimer’s and Parkinson’s remains unclear. In the brain, microglial cells migrate to sites of neural damage in response to neuro-inflammatory signals, and dispose of dying cells and insoluble cell debris by engulfing and enzymatically digesting them. The microglia therefore perform essentially the same role as that carried out by the immune cells known as macrophages in other tissues. However, neuro-inflammatory responses may also contribute to the pathogenesis of neurodegeneration, as microglia are known to be activated in virtually all types of dementia. This may simply relate to their role as phagocytic cells in the degradation of the extracellular protein deposits (amyloid plaques) that are a hallmark of Alzheimer’s. But it is also possible that activated microglia promote disease progression by secreting molecular signals that exacerbate inflammatory responses which are ultimately deleterious to healthy nerve cells.
The new study was carried out by an interdisciplinary German-Swiss team of cell biologists, radiologists and neuropathologists led by Professor Christian Haass, who holds the Chair of Metabolic Biochemistry at LMU and is Speaker of the German Center for Neurodegenerative Diseases (DZNE) in Munich. To clarify whether the microglia are the good guys or the bad guys, the researchers focused on the function of the gene TREM2. In the brain, this gene is expressed predominantly in microglia. Furthermore, mutations that impair its expression or the function of its protein product are associated with increased risk for neurodegenerative conditions such as Alzheimer’s, Parkinson’s and frontotemporal dementia (FTD).
With the aid of the CRISPR/Cas9 gene-editing system, Haass and his colleagues altered a single subunit (base-pair) in the coding sequence of the TREM2 gene of mice, which directs the synthesis of the TREM2 protein. In humans, this same mutation is associated with increased risk for a form of FTD. In earlier studies, it had been demonstrated that the normal TREM2 protein is transported to the cell membrane in order to perform its biological function. The mutation introduced by the CRISPR system disrupts this process, such that very little of the protein is expressed on the surface of microglial cells. In mice, this genetic alteration leads to a drastic impairment of microglial function, as evidenced by a variety of tests. For example, the mutant strain no longer activates its microglial cells in response to neuronal loss in the brain. As a result, the cells fail to migrate to sites of cell damage – and dead cells, insoluble debris and plaques cannot be disposed of. In addition, the mutation has catastrophic consequences for energy metabolism. The normal brain is totally dependent on glucose as an energy source, but loss of the TREM2 function leads to a significant fall in glucose consumption in the mutant brain. Moreover, the blood supply to the brain in a whole is markedly curtailed. Similar phenomena are observed in patients who carry loss-of-function mutations in the TREM2 gene. Taken together, these observations argue that microglial activation is indispensable for normal brain function.
For further details, please check the link here.





